Climate change has become one of the most pressing global challenges of our time. One of the main contributors to climate change is the increasing level of carbon dioxide (CO2) in the atmosphere, largely due to human activities such as burning fossil fuels. In response, researchers around the world are seeking effective and efficient ways to remove CO2 from the atmosphere and mitigate the effects of climate change. Among the potential solutions is the direct removal of CO2 from ocean water, which has been proposed as a promising approach. Recently, a team of researchers at MIT may have made a breakthrough in this field with the development of a new, low-cost, and efficient technique for removing CO2 from seawater. This article will explore the potential of this new technology and its possible implications for addressing the challenge of climate change.
Researchers from all over the world have spent years looking for effective ways to remove carbon dioxide (CO2) from the atmosphere. The planet’s main “sink” for atmospheric CO2 is the ocean, which absorbs 30 to 40 per cent of all gas produced by human activity. One potential way to reduce CO2 emissions and achieve net negative emissions is to extract CO2 directly from ocean water, a possibility that has been recently highlighted. However, the existing methods of removing CO2 from seawater require expensive membranes and chemicals to drive the electrode reactions at either end of the stack. A team of researchers from MIT has come up with a new process consisting of membrane-free electrochemical cells that are reversible and inexpensive.
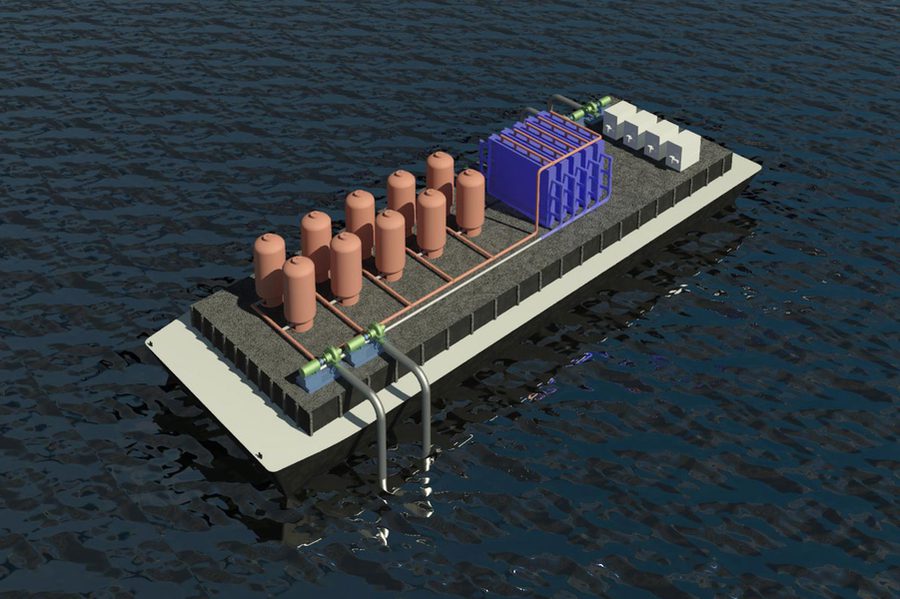
The team created a reversible process without membranes that uses reactive electrodes to release protons to seawater fed to the cells, driving the release of dissolved carbon dioxide from the water. The process is cyclic; it first acidifies the water to convert dissolved inorganic bicarbonates to molecular carbon dioxide, which is collected as a gas under vacuum. Then, the water is fed to a second set of cells with a reversed voltage to recover the protons and turn the acidic water back to alkaline before releasing it back into the sea. Once one set of electrodes is depleted of protons during acidification and the other set is regenerated during alkalization, the roles of the two cells are reversed.
Reinjecting the alkaline water and removing carbon dioxide can slowly reverse the acidification of the oceans caused by carbon dioxide accumulation. This, in turn, has threatened coral reefs and shellfish. The reinjection of alkaline water could be done through dispersed outlets or far offshore to avoid a local spike of alkalinity that could disrupt ecosystems.
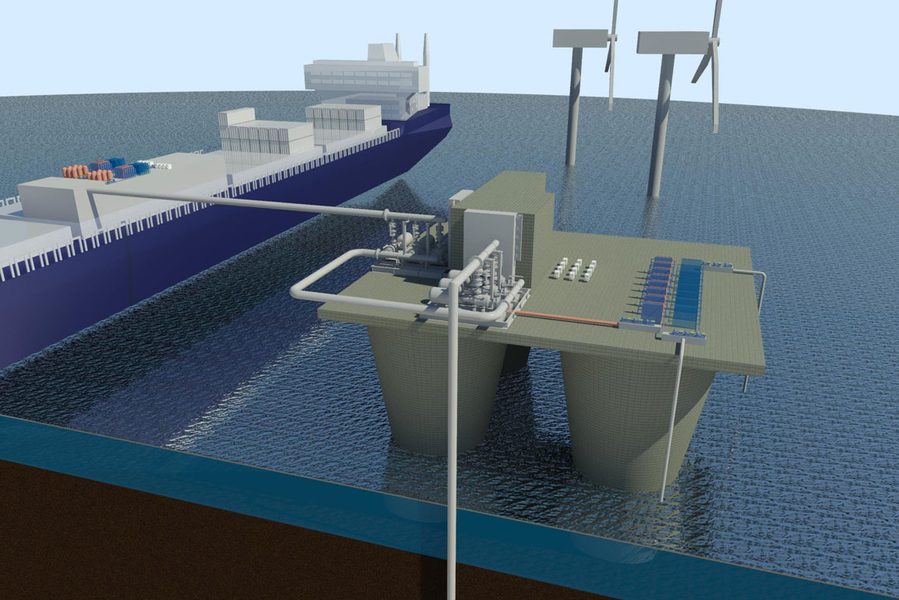
However, once the CO2 is removed from the water, it must be disposed of as with other carbon removal processes. For example, it can be buried in deep geologic formations under the sea floor, chemically converted into a compound like ethanol that can be used as a transportation fuel or into other specialty chemicals. “You can certainly consider using the captured CO2 as a feedstock for chemicals or materials production, but you’re not going to be able to use all of it as a feedstock,” says Hatton. “You’ll run out of markets for all the products you produce, so no matter what, a significant amount of the captured CO2 will need to be buried underground.”
While it is unlikely to be feasible to treat the entire planet’s emissions, the reinjection of alkaline water could be done in some places such as fish farms, which tend to acidify the water, to help counteract that effect.
- BC Approves Logging in 5,700 Hectares of Caribou Habitat as Population Drops 51% Since 1991
- Ontario Kills Endangered Species Act: Wildlife Protection Narrowed to Nests and Dens Only
- Histamine Alert: Vicente Marino Anchovy Recall Spans 6 Provinces After Confirmed Illnesses
- 8 Female Pacific Sleeper Sharks Form Orderly Queue at 1,629m Depth, Pushing Range 1,200km Further South
- Nipah Virus Returns to Kerala: 38-Year-Old Woman Tests Positive as 345 Contacts Monitored
The development of a new, reversible process for removing carbon dioxide from seawater, created by a team of researchers from MIT, holds great potential for mitigating the effects of carbon emissions on the environment. The membrane-free electrochemical cells offer a more affordable and sustainable solution compared to existing methods. By reinjecting alkaline water into the ocean, the process could counteract the acidification of the ocean and help protect coral reefs and shellfish. While not a complete solution to the problem of carbon emissions, the MIT team’s discovery is a significant step towards a more sustainable future.