UW researchers, utilizing post-mortem brain samples from 22 ADRC project participants, have unearthed pivotal insights on human microglia and their intricate relationship with Alzheimer’s disease.
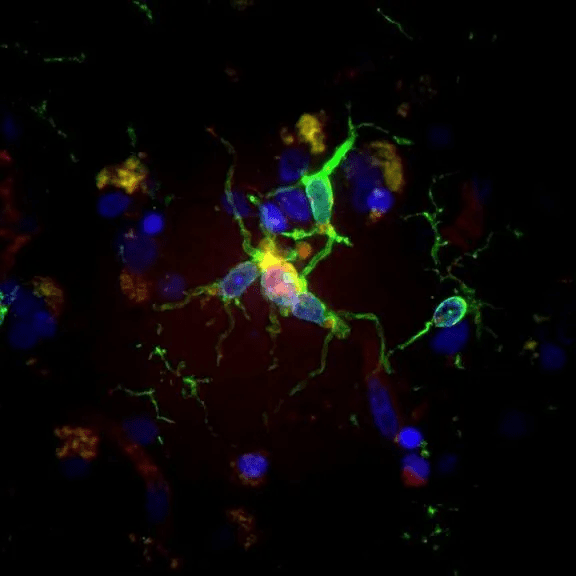
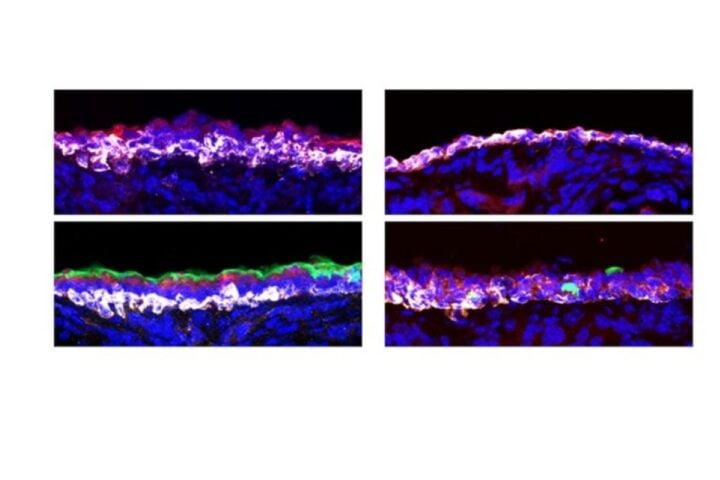
Microglia, vividly depicted in green, adopt a reactive shape when associated with amyloid-β deposits, showcased in purple. Alternatively, they assume a homeostatic shape in the parenchyma, distant from Aβ. Serving as the brain’s immune system, microglia’s primary role is paramount.
These cells undertake a multitude of tasks, with one of the most significant being the digestion of abnormal protein clumps intrinsically linked to Alzheimer’s disease.
Emerging evidence suggests that certain microglia cells might exhibit an exaggerated response in individuals with Alzheimer’s, instigating inflammation that further complicates the disease. As Katherine Prater, PhD, articulates, “The challenge lies in the fact that anti-inflammatory drugs tested haven’t shown promise.”
The Alzheimer’s community is currently engrossed in discussions about the potential of targeting specific microglia groups, aiming to mitigate the inflammatory environment characteristic of the disease.
The ADRC research team embarked on a comprehensive study to decipher the microglia groups that are responsive to Alzheimer’s disease. Their focus was predominantly on microglia gene expression, which orchestrates cell functionality through protein synthesis. Intriguingly, the researchers identified three microglia groups, previously uncharted in the human brain. “Discovering these new groups was a thrilling moment for our team,” shares Prater with palpable enthusiasm.
In individuals diagnosed with Alzheimer’s during their lifetime, a particular microglia group was more prevalent. This group’s gene expression bore signs of inflammation, hinting at a distinct response to Alzheimer’s stressors. To extract microglia from post-mortem brain samples, the research team employed an innovative adaptation of existing technology.
Their refined technique facilitated the extraction of a more substantial number of microglia cells, paving the way for a thorough examination of diverse cell groups. “Our findings in human brains align perfectly with predictions from animal and in vitro cell models of Alzheimer’s,” Prater notes. The team also delved into a microglia population, primarily responsible for maintaining brain functionality rather than waste disposal. Notably, they pinpointed a unique microglia subgroup, exclusive to Alzheimer’s brain tissue.
These microglia displayed evidence of intercellular communication, shedding light on potential Alzheimer’s mechanisms. “Our goal is to target only the harmful microglia types, rather than the entire immune cell population,” Prater emphasizes.
Similar Posts
With their recent advancements, the team is poised to delve deeper into the role of microglia in Alzheimer’s disease, armed with unparalleled precision. Their study, titled “Human microglia show unique transcriptional changes in Alzheimer’s disease,” found its place in the July 2023 issue of Nature Aging. Spearheaded by Prater, Green, Mamde, and a team of adept researchers, the study illuminates the intricate realm of microglia.
Microglia cells, in their response to Alzheimer’s proteins, are vividly captured in the study’s microscopic images, a testament to Alexandra Cochoit’s expertise. This research endeavor was made feasible through the generous funding from the NIA grant AG066509.
Microglia, the brain’s innate immune cells, play multifaceted roles that can influence the trajectory of this disease. This study zeroes in on the unique transcriptional changes in Alzheimer’s, spotlighting microglial populations intrinsically linked to the disease. The research offers profound insights, predicting the underlying gene regulatory networks. “Our approach to understanding Alzheimer’s is evolving, and microglia are at the forefront of this shift,” states Suman Jayadev.
The Nature Aging article accentuates the pivotal role of diverse microglia in Alzheimer’s progression. The research’s meta-description encapsulates the distinct transcriptional shifts in Alzheimer’s. The DOI serves as a gateway for readers seeking an in-depth exploration of the study.
The research, initially proposed in July 2022, received its approval in April 2023. It was formally unveiled on May 29, 2023, in the esteemed July issue of Nature Aging.
The article’s citation stands as a testament to the collaborative spirit of Prater, Green, Mamde, and their esteemed colleagues. The groundbreaking nature of the research is palpable, as it delves into the diversity of human microglia in Alzheimer’s.
The study’s revelations hold promise for the future, potentially offering targeted treatments for the millions grappling with Alzheimer’s. “The future of Alzheimer’s research is promising, and our findings are just the tip of the iceberg,” Prater concludes. As the scientific community continues its relentless pursuit of knowledge, microglia emerge as a beacon of hope in the battle against this formidable disease.