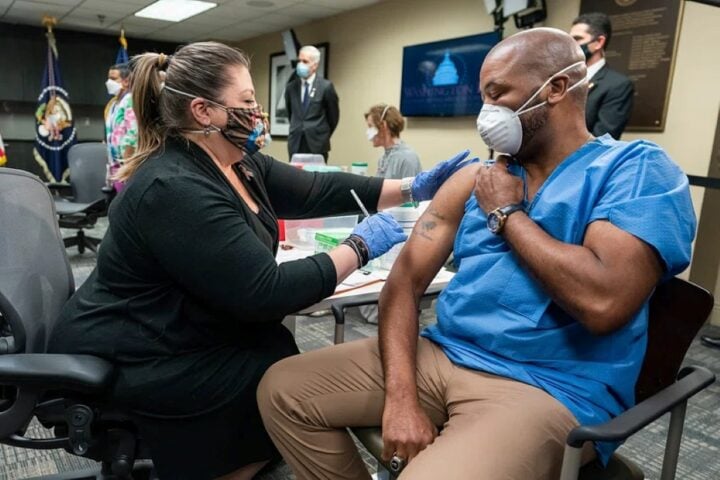
An Indian pharmaceutical company, Marion Biotech, has been implicated in a tragic incident where its cough syrups were linked to the deaths of 19 children in Uzbekistan due to poisoning. According to a report by Reuters, Marion Biotech, based in Noida, Uttar Pradesh, allegedly used industrial-grade ingredients in its cough syrups. The company purchased propylene glycol (PG) from Maya Chemtech India, a trader known for selling industrial-grade materials rather than pharmaceutical-grade ingredients. The cough syrups in question, Dok-1 Max and Ambronol, manufactured by Marion Biotech, were the subject of a global medical alert issued by the World Health Organization (WHO). The Indian government has launched an investigation into the company and suspended its license. Their syrups contained toxic industrial-grade PG. This industrial substance is commonly used in liquid detergents, antifreeze, paints, coatings, and pesticides.
Maya Chemtech, the supplier of PG, was revealed to lack a license to sell pharmaceutical-grade materials. It is reported that the cough syrup was manufactured using this toxic ingredient. The Pharmaceutical Export Promotion Council of India (Pharmexcil) has consequently suspended the registration certificate of Marion Biotech Pvt. Ltd, the producer of the cough syrup linked to the deaths of 18 children in Uzbekistan. This incident highlights a larger concern regarding the quality of drugs exported from India, as the country is the largest exporter of generic drugs worldwide. The WHO recently flagged seven India-made syrups linked to over 300 deaths globally, while approximately 20 syrups manufactured by Indian and Indonesian companies were also flagged. The reputation of the Indian pharmaceutical industry and the trust of international agencies in Indian pharma exports have been negatively impacted.
Marion Biotech’s membership with Pharmexcil, a body established by the Ministry of Commerce and Industry to promote drug exports, has been suspended due to the company’s failure to provide requested information about the toxic cough syrup. As a result, Marion Biotech’s export capabilities have been significantly affected. The Indian government has initiated an inquiry into the matter, with officials from the Central Drugs Standard Control Organisation (CDSCO) and the Uttar Pradesh drug regulator visiting Marion Biotech’s facility in Noida to collect drug samples for testing. All manufacturing activities at the plant have been ordered to cease during the investigation. Pharmexcil, in a letter to Marion Biotech’s chairman and managing director Sachin Jain, stated that the Agency on Development of Pharmaceutical Industries in Uzbekistan had taken serious note of the incident. It was discovered that the batch of drugs contained ethylene glycol at a level 300 times higher than permitted by medical regulations, while the correct composition should have included propylene glycol.
Similar Post
The tragic consequences of supplying substandard medicines have tarnished the reputation of the Indian pharma industry and may lead to diminished trust from international agencies regarding Indian pharma exports, as stated in the letter from Udaya Bhaskar, director general of Pharmexcil. The incident in Uzbekistan follows a similar controversy involving the deaths of 66 children in Gambia allegedly due to contaminated cough syrup. The concerns raised about the quality of exported drugs have prompted the Indian government to take action and launch an inquiry. Uzbekistan, the 44th largest export destination for India, holds significant importance in the CIS region for pharmaceutical items. In FY 21-22, pharmaceutical exports from India to Uzbekistan amounted to $142 million, and from April to November of FY 22-23, exports were reported at $84.6 million. The impact of this incident on the Indian pharma industry and its export market remains a significant concern. Previously, another Indian pharmaceutical company, Global Pharma Healthcare PVT Limited, was in the news for their eye drops that caused vision loss and other side effects in the US.