When Poppy was born, doctors told her mother Kat there was no cure for mitochondrial disease. Now 14, Poppy uses a wheelchair, cannot speak, and relies on tube feeding. But for families like the Kittos, eight healthy babies born in the UK represent a turning point in preventing one of medicine’s most cruel genetic conditions.
Published findings in the New England Journal of Medicine confirm that eight children—four boys and four girls, including twins—have been born free of mitochondrial disease using pronuclear transfer, a technique that combines DNA from three people. All eight children were healthy at birth, meeting their developmental milestones, with disease-causing mitochondrial DNA mutations either undetectable or present at levels very unlikely to cause disease.
How Three People Create One Healthy Baby
Mitochondria function as cellular powerhouses, converting food into energy through a process requiring oxygen. These tiny structures contain their own DNA separate from nuclear DNA, inherited solely from mothers to children. Around one in 5,000 children is born with mitochondrial DNA mutations that can cause devastating disease.
Mitochondrial disorders affect organs with high energy demands—brain, heart, and muscles—causing symptoms ranging from seizures and developmental delays to organ failure and early death. Unfortunately, there is no cure for mitochondrial disease at present, with treatment usually supportive, relieving symptoms that develop.
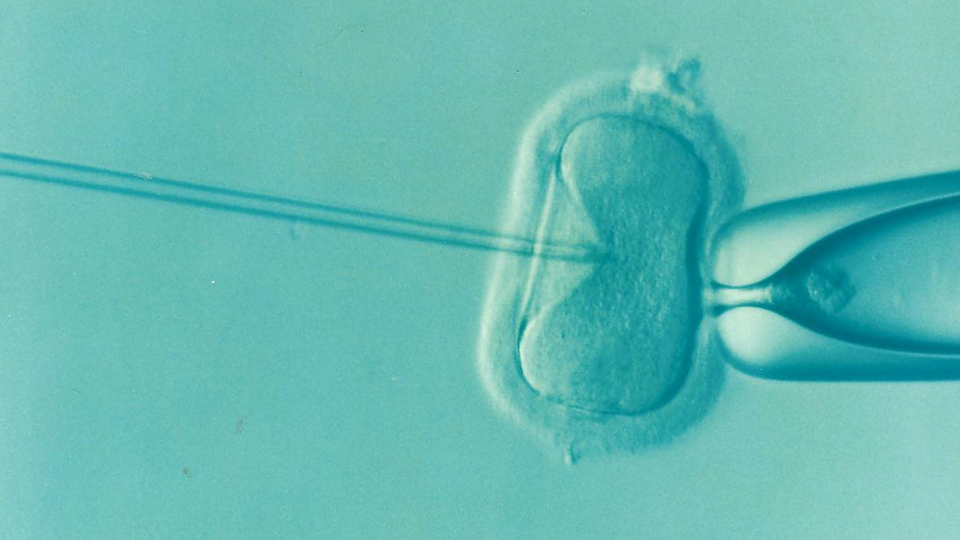
Pronuclear transfer works by creating embryos from both intended parents and a donor, then transferring the nuclear DNA (containing traits like eye color and height) from the parents’ fertilized embryo into a donor embryo that has healthy mitochondria but has had its nuclear DNA removed. The pronuclei from the patient’s egg are then allowed to fuse with the donor egg, resulting in a reconstructed egg containing nuclear DNA from the patient and her partner and mitochondrial DNA from the unaffected donor.
The resulting child carries 99.9% of their genetic material from their biological parents, with less than 0.1% from the mitochondrial donor—too small to influence appearance, personality, or other inherited traits.
Clinical Success Rates and Safety Data
The Newcastle University team treated 22 women, resulting in eight healthy babies and one ongoing pregnancy. This represents a clinical pregnancy rate of approximately 36%.
Levels of disease-causing mitochondrial DNA detected in babies born after pronuclear transfer treatment ranged from undetectable to 16% in neonatal blood. Three of the babies had levels of disease-causing mitochondrial DNA mutations of up to 16 percent, which is still below the 80 percent threshold for clinical disease.
One of the eight babies experienced infant myoclonic epilepsy, which resolved spontaneously, while another child has cardiac arrhythmia responding to treatment. These conditions are not believed to be linked to the mitochondrial replacement therapy.
Regulatory Framework and Patient Access
As of July 1, 2025, 35 patients have been given approval for mitochondrial donation treatment by the HFEA Statutory Approvals Committee. These decisions are made on an individual case by case basis where there are no other options for the families involved and in strict accordance with the law.
The UK Human Fertilisation and Embryology Authority (HFEA) oversees each application individually. Currently, only Newcastle Fertility Centre at Life has a licence to conduct research and treat patients using mitochondrial donation techniques.
NHS England allocated £8 million over five years to fund treatment costs, with a Department of Health impact assessment estimating discounted £318 million over 10 years in potential net benefits.
Global Regulatory Landscape
The UK pioneered both the science and regulation of mitochondrial replacement. Parliament passed The Human Fertilisation and Embryology (Mitochondrial Donation) Regulations in 2015, making the UK the first country to legalize the procedure.
Australia became the second country to allow clinical use through Maeve’s Law, passed in 2022 and named after a Melbourne girl with severe mitochondrial disease. The mitoHOPE team aims to start recruiting into clinical trials in 2025, pending ethics approval.
The United States maintains a Congressional ban, with provisions in annual funding bills preventing the Food and Drug Administration from accepting applications for research involving heritable embryo modifications.
Historical Context: The Mexico Case
In 2016, Dr. John Zhang and his team used spindle transfer to help a Jordanian woman give birth to a baby boy, representing the first successful mitochondrial replacement birth. The mother had Leigh syndrome and had experienced four miscarriages and lost two children to the disease previously.
Similar Posts
The procedure was performed in Mexico because US laws effectively prohibited the technique, with Zhang stating he went there because “there are no rules”. In August 2017, the FDA issued a warning letter to Zhang’s clinic for marketing the technique without authorization.
Expert Commentary and Scientific Assessment
Peter Thompson, Chief Executive of the HFEA, said: “For the first time, families with severe inherited mitochondrial illness have the possibility of a healthy child”.
Professor Mary Herbert, lead author of the reproductive outcomes paper, stated: “The findings give grounds for optimism. However, research to better understand the limitations of mitochondrial donation technologies will be essential to further improve treatment outcomes”.
Professor Nils-Göran Larsson from Karolinska Institute described the study as “very important and represents a breakthrough in mitochondrial medicine,” while emphasizing the need for long-term follow-up studies.
Some experts noted concerns about the timeline, questioning why eight births occurred over seven years when earlier projections suggested 150 yearly births if all eligible women chose the procedure.
International Clinical Experience
In 2019, Greek and Spanish researchers announced successful pregnancies using spindle transfer for women with repeated IVF failures, with six babies born in a pilot study of 25 infertile couples. Pilot follow-up reports indicated the children remain healthy.
About 30 babies worldwide were born using cytoplasmic transfer in the late 1990s, but the FDA halted this earlier technique in 2001 after safety concerns emerged.
Economic and Social Impact
The Department of Health’s impact assessment projected potential cost savings to health and social welfare systems through reduced disease burden, though detailed economic modelling has not yet been published.
MRT costs vary significantly in private settings, though NHS provision makes it accessible to approved UK patients.
Technical Challenges and Future Improvements
Carryover of maternal mitochondrial DNA remains a known limitation, with the Newcastle team seeking to better understand and address this issue as part of ongoing research. Optimization of protocols reduced mtDNA carryover to less than 2% heteroplasmy in 79% of blastocysts in preclinical studies.
Australian regulations define five different mitochondrial donation techniques: maternal spindle transfer, pronuclear transfer, first polar body transfer, second polar body transfer, and germinal vesicle transfer, offering multiple technical approaches.
Patient Experiences and Family Impact
“After years of uncertainty this treatment gave us hope—and then it gave us our baby,” said the mother of a baby girl born through the technique. “We look at them now, full of life and possibility, and we’re overwhelmed with gratitude”.
Another mother stated: “We are now proud parents to a healthy baby—a true mitochondrial replacement success. This breakthrough has lifted the heavy cloud of fear that once loomed over us”.
Long-term Monitoring and Follow-up
All babies born through mitochondrial donation undergo intensive health monitoring, with the HFEA continuing to review clinical and scientific developments. Experts stress the importance of long-term follow-up, potentially including multi-generational data collection to detect effects that might appear later in life.
The Newcastle team recommends that patients undergoing pronuclear transfer consider antenatal screening for mitochondrial disease for additional reassurance, as they cannot yet guarantee complete elimination of disease risk.
Research and Development Pipeline
Australia’s mitoHOPE program, funded with $15 million from the Medical Research Future Fund, aims to build evidence of safety, efficacy, and feasibility while refining available techniques. The Australian team plans to begin recruiting participants in 2025.

The Newcastle team has developed an integrated program involving both preimplantation genetic testing and pronuclear transfer, with patients with heteroplasmy offered PGT while those with homoplasmy or elevated heteroplasmy offered pronuclear transfer.
The documented evidence shows that pronuclear-transfer mitochondrial donation has led to eight healthy births under UK regulation, with low residual mutant mtDNA levels and a 36% pregnancy rate. Licences are issued case-by-case under 2015 legislation, with 35 patients receiving approval. Scientific literature identifies carry-over minimisation, heteroplasmy thresholds, and long-term monitoring as ongoing priorities, while costs, alternative techniques, and international regulatory frameworks continue to define access parameters.