MIT engineers have developed a breakthrough in hydrogen separation technology that could make hydrogen fuel production cheaper and more efficient. Their new palladium membrane design can withstand much higher temperatures than conventional versions, potentially transforming how we produce clean hydrogen energy.
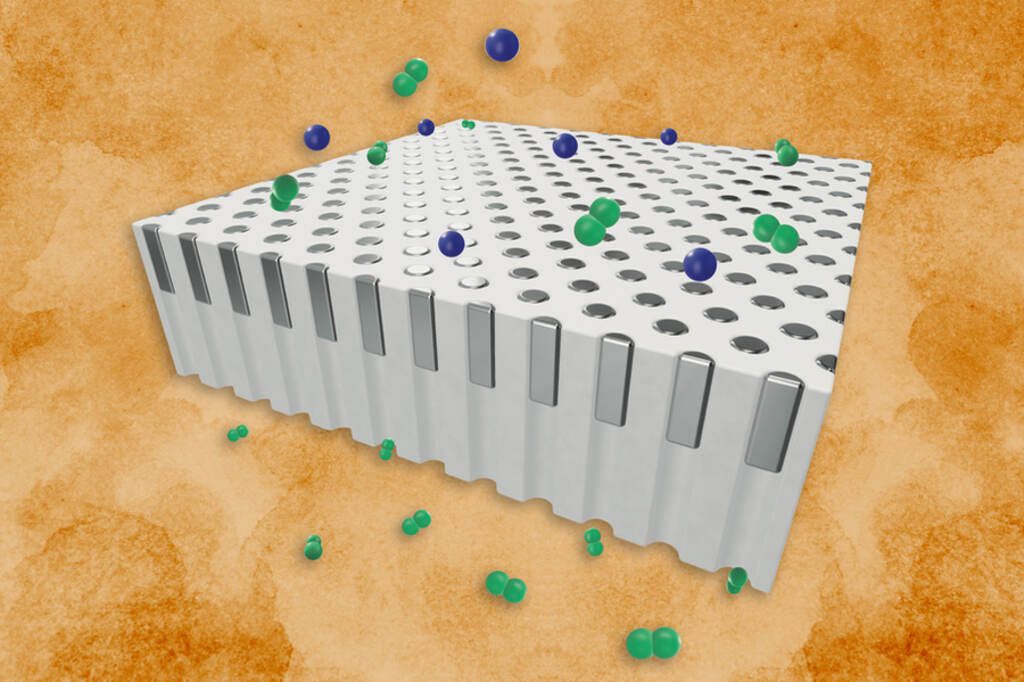
Palladium is a silvery metal with a unique ability to act as a natural filter for hydrogen. It blocks all other gases while letting only hydrogen pass through. This remarkable selectivity makes palladium membranes essential in industries that need pure hydrogen, including semiconductor manufacturing, food processing, and fertilizer production.
The problem with traditional palladium membranes is that they break down at high temperatures above about 800 Kelvin (about 527°C). This limitation has forced hydrogen production systems to include costly cooling steps before separation can occur.
Similar Posts
The MIT team tackled this problem with an innovative design approach. Instead of using palladium as a continuous film, which is standard practice, they deposited palladium as tiny “plugs” into the pores of a supporting material. Each pore in the silica support measures about half a micron wide – smaller than a human red blood cell.
“With further work on scaling and validating performance under realistic industrial feeds, the design could represent a promising route toward practical membranes for high-temperature hydrogen production,” says Lohyun Kim, former graduate student at MIT and lead researcher on the project.
The new plug-based design shows remarkable heat resilience. In tests, the membranes remained stable and kept separating hydrogen from other gases even after 100 hours at 1,000 Kelvin (about 727°C). This represents a 200 Kelvin improvement over conventional membranes.
This temperature boost matters because it could simplify hydrogen production from processes like steam methane reforming and ammonia cracking. Both methods operate at high temperatures, and until now, the gases needed cooling before hydrogen could be separated. The new membranes could potentially work directly in the hot reaction zone, eliminating cooling equipment and reducing system complexity.
In practical terms, this means future hydrogen production facilities could become more compact and energy-efficient. The technology could enable “membrane reactors” where methane gas would flow directly through a chamber, with the membrane filtering out pure hydrogen in one step – replacing today’s multi-stage process.
The research team notes that more work is needed before commercial use. The membrane must be scaled up from lab-sized samples, and its performance needs testing under realistic industrial conditions with various gas mixtures and potential contaminants.
The research was funded by Eni S.p.A. through the MIT Energy Initiative and was published in the journal Advanced Functional Materials.