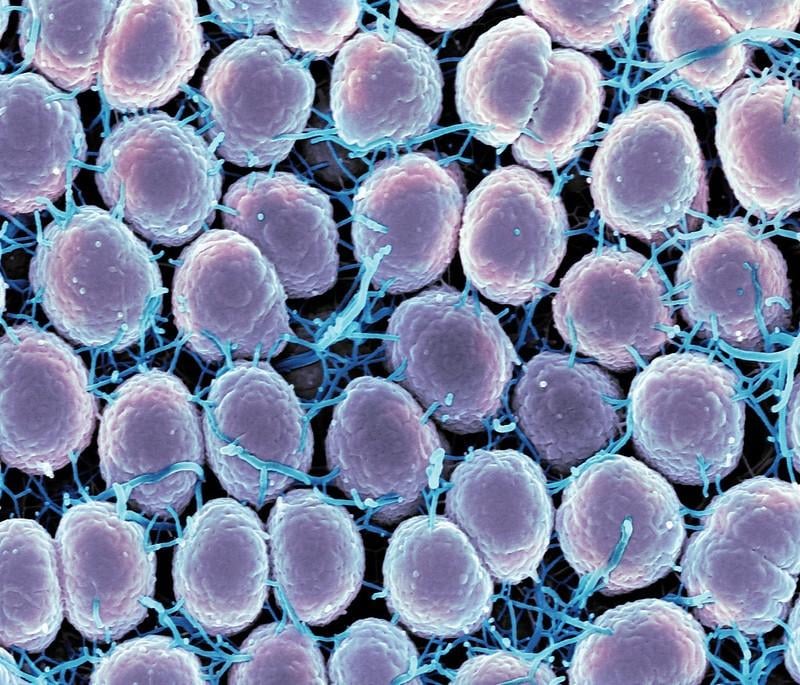
England is set to become the first country in the world to offer a routine vaccination programme against gonorrhoea, with the rollout beginning in August 2025. This breakthrough initiative comes as gonorrhoea cases in England reached 85,000 in 2023 — the highest figure since records began in 1918 and triple the number reported in 2012.
The programme will use an existing meningitis B vaccine called 4CMenB (Bexsero) that scientists discovered can also protect against gonorrhoea. This cross-protection works because the bacteria causing meningitis B and gonorrhoea share genetic similarities.
Why England, Why Now?
The UK Health Security Agency (UKHSA) reported 17 cases of ceftriaxone-resistant gonorrhoea and 9 extensively drug-resistant cases between January 2024 and March 2025. These alarming figures highlight the growing threat of antibiotics becoming ineffective against the infection.
“Not only will this rollout provide much needed protection to those that need it most, but it will make the UK the first country in the world to offer this protection and a world leader in protecting people against gonorrhoea,” said Dr. Sema Mandal, NHS Consultant Epidemiologist and Deputy Director at UKHSA.
Studies show the vaccine offers between 30-42% protection against gonorrhoea. While not providing complete immunity, analysis from Imperial College London suggests the programme could prevent up to 100,000 cases over the next decade and save the NHS approximately £7.9 million.
Similar Posts
Why Isn’t the EU Following Suit?
Despite rising gonorrhoea cases across Europe, the EU has not announced similar vaccination programmes. This divergence appears to stem from different approaches to public health innovation and risk assessment.
The UK’s Joint Committee on Vaccination and Immunisation (JCVI) advised in November 2023 that even with modest effectiveness (around 32-42%), the vaccine was worth implementing given the severity of the gonorrhoea crisis and growing antibiotic resistance.
While the available documents don’t explicitly state the EU’s position, the absence of similar programmes suggests European health authorities may be waiting for vaccines with higher effectiveness rates or conducting their own cost-benefit analyses.
Who Will Get the Vaccine?
The NHS programme will target those at highest risk of infection, primarily:
- Gay and bisexual men and other men who have sex with men who have a recent history of multiple sexual partners or STIs
- Other groups at equivalent risk, including sex workers and people with histories of bacterial STIs
Local authority-commissioned sexual health services will identify eligible individuals and invite them for vaccination. At these appointments, patients will also be offered vaccines for mpox, Hepatitis A and B, and Human Papillomavirus (HPV).
“By targeting those most at risk, we can reduce transmission rates from this unpleasant disease that is becoming harder to treat and prevent thousands of cases over the next few years,” said Ashley Dalton, Minister for Public Health and Prevention.
The Bigger Picture: Fighting Antibiotic Resistance
The vaccination programme represents more than just STI prevention — it’s part of the global fight against antibiotic resistance. Gonorrhoea has shown remarkable ability to develop resistance to antibiotics, raising fears of “super gonorrhoea” strains that may eventually become untreatable.
“This is excellent news and a landmark moment for sexual health in England,” said Professor Matt Phillips, President of the British Association for Sexual Health and HIV. “Gonorrhoea diagnoses are at their highest since records began and this has the potential to help us to turn that around.”
Beyond the UK’s vaccine initiative, pharmaceutical companies are racing to develop new treatments. GSK’s gepotidacin and another oral antibiotic called zoliflodacin are currently in clinical trials, showing promise against drug-resistant strains.
Several companies are also working on dedicated gonorrhoea vaccines that may offer higher protection rates in the future. GSK’s investigational Neisseria gonorrhoeae vaccine received Fast Track designation from the US FDA in June 2023 and is in early clinical trials.

Richard Angell, Chief Executive of Terrence Higgins Trust, called the UK initiative a “game changer” that “could cut 40% of new gonorrhoea cases.”
The programme will begin offering vaccines from early August, marking a significant shift in how countries approach sexually transmitted infections in an era of increasing antibiotic resistance.