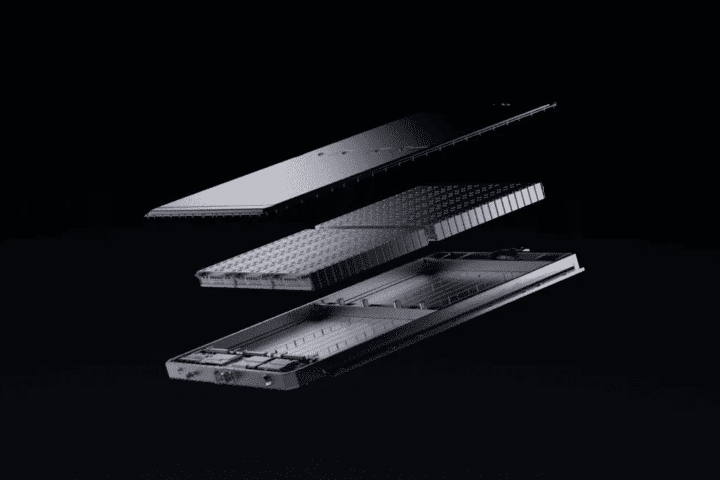
MIT engineers have developed a new sustainable technology for making clean hydrogen fuel using everyday aluminum cans and ordinary seawater. Their research shows this process produces a fraction of the carbon emissions compared to traditional hydrogen production methods, putting it on par with other green hydrogen technologies.
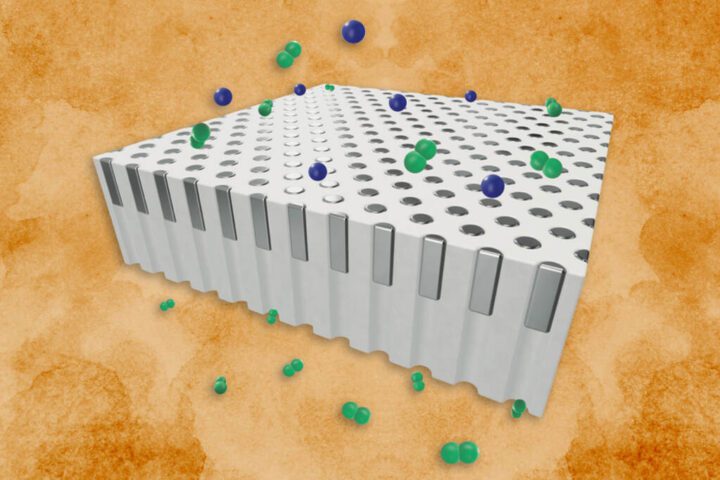
The discovery builds on decades-old chemistry with a practical twist. When pure aluminum touches water, it breaks water molecules apart to form hydrogen gas—a potential clean energy source that produces zero emissions when used. The MIT team has made this reaction practical and scalable as a sustainable technology.
“We’re showing a new way to produce hydrogen fuel, without carrying hydrogen but carrying aluminum as the ‘fuel,'” explains Aly Kombargi, who recently completed his PhD at MIT and led the research.

How It Works: Simple Chemistry with a Clever Hack
Aluminum naturally forms a protective shield when exposed to air, which prevents it from reacting with water. MIT researchers discovered they could overcome this barrier by treating aluminum pellets with a small amount of gallium-indium alloy. This “activates” the aluminum, allowing the pure metal underneath to react with water.
What makes this approach especially smart is that when the reaction happens in seawater, the salt ions help recover the gallium-indium afterward. This creates a cycle where the rare metal alloy—which is expensive—can be collected and reused repeatedly.
The team published their findings in Cell Reports Sustainability (June 2025), where they conducted a full life-cycle assessment of their process. For every kilogram of hydrogen produced, the entire operation—from recycling aluminum to transporting the fuel—would generate just 1.45 kilograms of carbon dioxide. For comparison, hydrogen made from fossil fuels emits around 11 kilograms of carbon dioxide per kilogram of hydrogen.
Economics and Real-World Applications
Cost remains competitive with other green hydrogen technologies at about $9 per kilogram. The team has already built working prototypes of this sustainable technology, including a small reactor about the size of a water bottle that can power an electric bike for several hours.
Maritime uses look particularly promising. As Kombargi states in MIT’s news release: “This is very interesting for maritime applications like boats or underwater vehicles because you wouldn’t have to carry around seawater—it’s readily available. We also don’t have to carry a tank of hydrogen. Instead, we would transport aluminum as the ‘fuel,’ and just add water to produce the hydrogen that we need.”
The researchers have calculated that a reactor holding about 18 kg (40 pounds) of aluminum pellets could power a small underwater glider for about 30 days by pumping in surrounding seawater.
Boehmite: The Valuable Byproduct
An important aspect of this sustainable technology is the production of boehmite—an aluminum-based mineral formed as a byproduct of the hydrogen generation process. Boehmite has commercial value in multiple industries.
The boehmite produced from this process could potentially create an additional revenue stream, helping to offset the overall cost of hydrogen production. However, it’s worth noting that industrial applications often require higher purity levels than what might be produced in this process.

Supply Chain Challenges
One significant hurdle for scaling this sustainable technology involves the gallium-indium supply chain. China currently produces about 80% of the world’s raw gallium, creating a potential vulnerability.
This dominance came about indirectly through China’s expansion in aluminum production. Since gallium is primarily extracted as a byproduct of processing bauxite (the main ore for aluminum), China’s tenfold increase in aluminum production between 2000 and 2022 gave it control over much of the gallium supply chains.
In July 2023, China implemented export restrictions on gallium, demonstrating the potential for supply disruptions. While permits are currently being granted, this highlights the need for supply chain diversification if the technology is to scale globally.
The Caffeine Connection
In their 2024 research, the MIT team made a surprising discovery—adding a small amount of caffeine significantly speeds up the hydrogen-producing reaction. They found that imidazole—an active ingredient in caffeine—reduced reaction time from two hours to just five minutes. This led researchers to experiment with adding coffee grounds to the mixture, which successfully accelerated hydrogen production.

Practical Implementation and Challenges
The researchers envision a hydrogen supply chain very different from today’s systems. In their model:
- Scrap aluminum from recycling centers would be processed into pellets and treated with gallium-indium
- These pretreated pellets would be shipped as solid “fuel” to coastal hydrogen stations
- At these stations, the pellets would mix with seawater on demand to produce hydrogen
- Consumers could pump the hydrogen directly into vehicles with either combustion engines or fuel cells
This approach solves two major hydrogen challenges: transportation safety and energy density. Aluminum can store hydrogen potential at a density approximately ten times higher than compressed gas, making it safer and more efficient to transport.

However, several practical challenges remain:
- Aluminum quality: Not all recycled aluminum is equal—contamination with coatings or plastics can affect hydrogen yield
- Recovery efficiency: The gallium-indium recovery process must be highly efficient to be economically and environmentally viable
- Material constraints: The limited global production of gallium and indium could present scaling challenges that would require increased production
Future Outlook
The MIT team continues refining their sustainable technology and exploring new applications. They’re currently developing advanced reactors and investigating underwater uses that would harness surrounding seawater to power boats or submersible vehicles.
“The process works, which is the most exciting part,” says Kombargi. “And we show that it can be environmentally sustainable.”
This research represents a promising approach to making hydrogen a practical, low-carbon fuel option. By turning everyday waste products like soda cans into clean energy, this sustainable technology offers an innovative path to reduce carbon emissions while addressing waste management challenges.
The environmental impact appears quite positive, though the carbon footprint calculations assume certain conditions such as relatively short transportation distances for aluminum scrap. If transport distances increase or if gallium-indium is sourced from primary ores rather than recycled, emissions could rise.
As with many emerging sustainable technologies, real-world implementation will likely reveal challenges and opportunities that further refine this promising approach to clean hydrogen production.