MIT researchers have uncovered a fundamental principle that could revolutionize battery technology, potentially leading to faster charging times and longer-lasting power for everything from smartphones to electric vehicles.
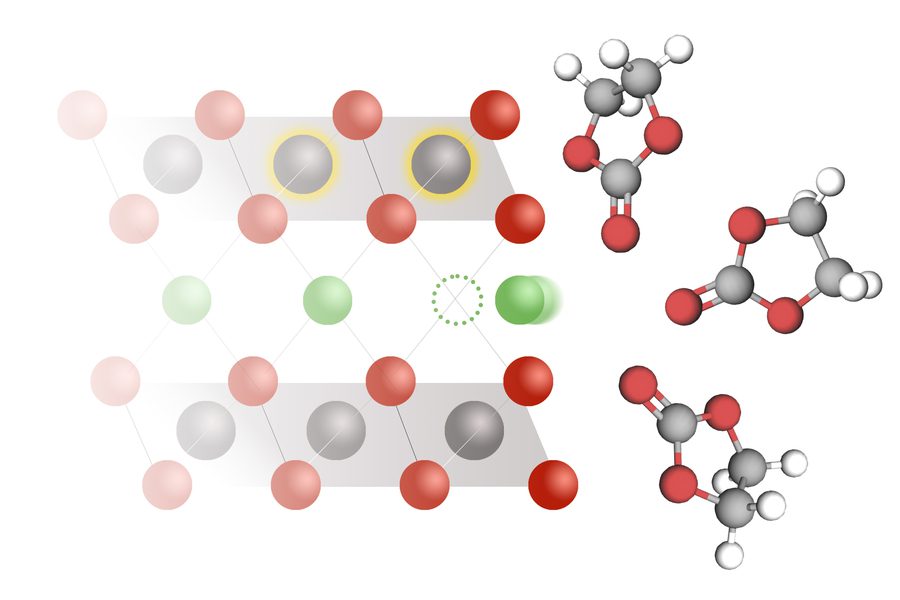
The team measured lithium intercalation rates in over 50 electrolyte–electrode combinations and found that the long-established model for how lithium ions enter battery electrodes was inaccurate. Their new model, called coupled ion-electron transfer (CIET), better explains what actually happens during charging and discharging.
“What we hope is enabled by this work is to get the reactions to be faster and more controlled, which can speed up charging and discharging,” says Martin Bazant, professor of chemical engineering and mathematics at MIT.
At the core of every lithium-ion battery is a simple process: lithium ions move from the electrolyte solution into a solid electrode when the battery discharges, and back to the electrolyte when charging. How quickly this happens determines charging speed and power output.
For decades, scientists believed this process followed the Butler-Volmer equation, developed almost a century ago. However, when researchers measured these rates in laboratories, results varied wildly—sometimes differing by a factor of one billion between labs.
The MIT team discovered that instead of moving independently, lithium ions and electrons work together. During charging, an electron must transfer to the electrode simultaneously with the lithium ion.
“The electrochemical step is not lithium insertion, which you might think is the main thing, but it’s actually electron transfer to reduce the solid material that is hosting the lithium,” Bazant explains. “Lithium is intercalated at the same time that the electron is transferred, and they facilitate one another.”
Similar Posts
The CIET model could guide engineers in designing better batteries without relying on trial and error.
The researchers tested materials used in everyday devices, including lithium nickel manganese cobalt oxide (found in electric vehicles) and lithium cobalt oxide (used in smartphones and laptops). Their measurements showed much slower reaction rates than previously reported—matching predictions from their new CIET model rather than the traditional Butler-Volmer approach.
Perhaps most exciting is that battery performance could be improved by changing the electrolyte composition. Simple adjustments like swapping different anions can lower the energy needed for the lithium-electron transfer, making the process more efficient.
“Tuning the intercalation kinetics by changing electrolytes offers great opportunities to enhance the reaction rates, alter electrode designs, and therefore enhance the battery power and energy,” says Yang Shao-Horn, professor of engineering at MIT.
The research could help reduce unwanted side reactions that degrade batteries over time. When electrons leave the electrode and dissolve into the electrolyte, they cause battery capacity to fade—a common reason why older phones don’t hold a charge like they once did.
Published in the prestigious journal Science, this research was funded by Shell International Exploration and Production and the Toyota Research Institute through the D3BATT Center, which focuses on data-driven battery design.
By providing a scientific framework for understanding how lithium ions and electrons move together, this discovery could help engineers systematically improve battery performance rather than relying on guesswork—potentially leading to electric vehicles that charge faster and phones that last longer between charges.