The CDC released a warning to the public on January 20th regarding the usage of EzriCare Artificial Tears eye drops. They advise healthcare providers and consumers to cease usage immediately. The CDC is conducting an investigation as 55 infections, resulting in permanent vision loss, hospitalization, and even one death, have been reported across 12 states in the US. Those who have sought medical attention have reportedly used one or more out of the over ten available brands of artificial tears, with the majority of the cases involving EzriCare’s product.
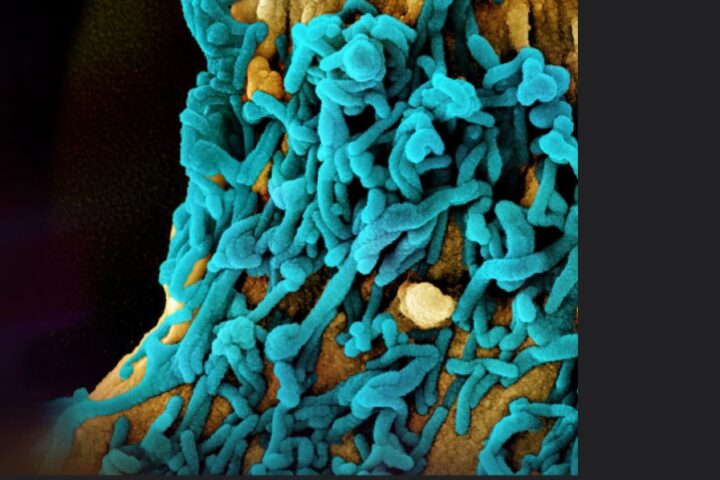
The infections associated with the EzriCare Artificial Tears eye drops have affected various areas such as the cornea, respiratory tract, urinary tract, intraocular fluids, and sepsis. Unlike other eye drop products, the EzriCare drops lack preservatives that would prevent bacterial growth. In response to the investigation, the CDC discovered that open bottles of EzriCare tested positive for Pseudomonas aeruginosa bacteria, which are highly resistant to antibiotics. Upon learning of the investigation, the New Jersey-based company promptly took action to halt the distribution and sale of the product. In addition, the company is reaching out to customers to advise against further use of the product to the greatest extent possible.
According to NBC News, the CDC has issued a warning for consumers who have used the EzriCare Artificial Tears eye drops and are experiencing symptoms such as yellow, green, or clear discharge from the eye, pain or discomfort, redness of the eye or eyelid, the sensation of having something in the eye, increased light sensitivity, or blurred vision. As of February 2nd, the product appears to have been recalled as it is no longer available on Amazon. The product was manufactured in India and is sold under different brand names in other countries. EzriCare, in collaboration with the US FDA, is working to recall the product and stated that the manufacturer, Global Pharma Healthcare PVT Limited, is also assisting with the recall effort. Additionally, the same product is marketed under different brand names.