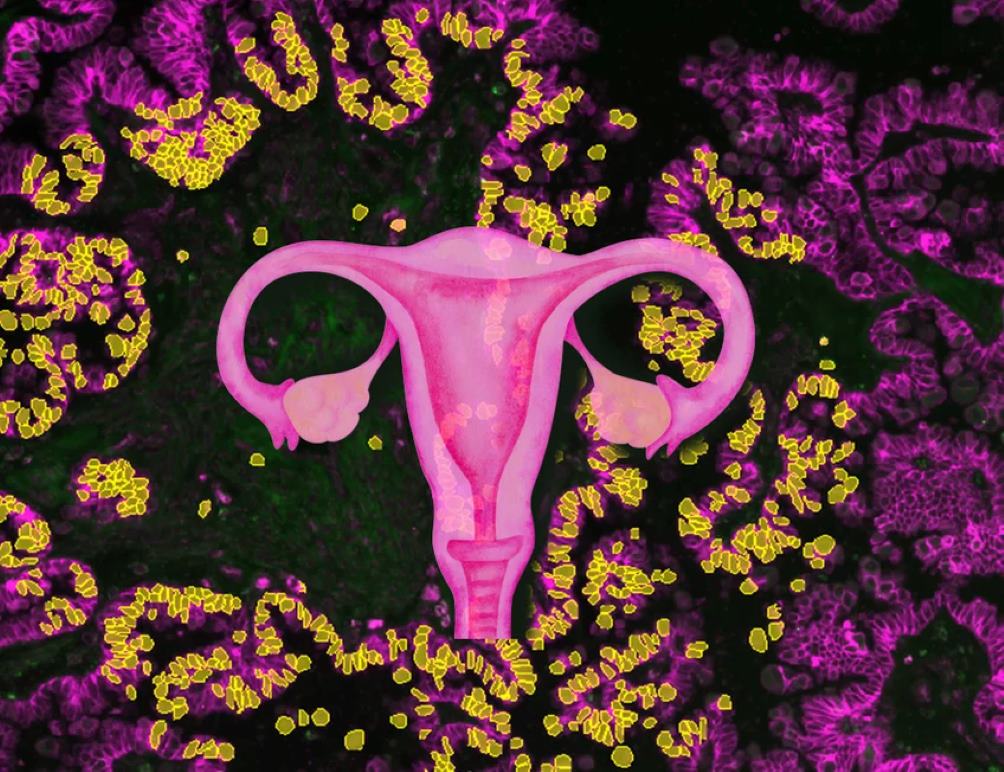
Low-grade serous ovarian cancer (LGSC) has long been one of the most challenging forms of ovarian cancer to treat. It affects younger women, resists standard chemotherapy, and often leads to late recurrences. But a new study brings hope, revealing how these tumors develop and identifying promising treatment combinations.
Scientists from the Max Planck Institute of Biochemistry and the University of Chicago have mapped how benign borderline tumors progress to invasive cancer. Using advanced technology called Deep Visual Proteomics, they’ve created detailed molecular maps showing precisely how tumors evolve within tissue environments.
“For gynecologic oncologists, metastatic low-grade serous ovarian cancer is one of the most challenging cancers to treat – patients are young when they get sick and it is resistant to chemotherapy,” explains Ernst Lengyel, gynecological oncologist involved in the research.
Traditional treatment for ovarian cancer has remained largely unchanged for decades. Surgery to remove visible cancer tissue combined with platinum-based chemotherapy has been the standard approach since the early 1990s. This typically involves six rounds of chemotherapy lasting 18-21 weeks, with patients experiencing significant side effects including neuropathy, fatigue, and immune suppression. While high-grade serous ovarian cancer often responds initially to this treatment, LGSC shows response rates below 15%.
“Unlike high-grade cancers, low-grade ovarian cancers do not respond well to conventional chemotherapy and may respond better to aggressive surgery and endocrine therapy, and possibly other targeted therapies,” notes researchers at the University of Chicago Medicine.
The new research identified 16 potential drug targets and tested a novel combination therapy: Milciclib paired with Mirvetuximab. This combination attacks cancer through two different mechanisms. Milciclib inhibits cyclin-dependent kinases, blocking cell division, while Mirvetuximab delivers a toxic payload specifically to cancer cells expressing the FOLR1 protein.
Lisa Schweizer, first author of the study, explains, “By profiling the disease at high spatial resolution, we could trace how these tumors evolve along a gradient of malignancy and interact with their environment.”
Similar Posts
In mouse models, this combination significantly reduced tumor burden. This targeted approach represents a dramatic shift from traditional treatments by focusing on specific molecular vulnerabilities rather than broadly attacking all rapidly dividing cells.
Mirvetuximab received FDA approval in March 2024 for folate receptor-positive, platinum-resistant epithelial ovarian cancer. Clinical trials showed a 42.3% response rate compared to just 15.9% with standard chemotherapy. Even more significant, overall survival increased from 12.75 months with chemotherapy to 16.46 months with Mirvetuximab.
The treatment regimen for Mirvetuximab consists of an intravenous infusion once every three weeks until disease progression or unacceptable toxicity. Studies show the duration of response averages about 6.9 months, significantly better than the 3-4 months typically seen with standard chemotherapy.
Cost remains a significant hurdle. While specific pricing for the Milciclib-Mirvetuximab combination isn’t yet available, cost-effectiveness studies for Mirvetuximab alone found it’s not cost-effective at its current price. Economic analyses suggest a 70-73% reduction in cost would be needed to meet standard cost-effectiveness thresholds in the U.S. healthcare system.
Professor Matthias Mann states, “This study demonstrates the transformative power of MS-based spatial proteomics in cancer research. By mapping thousands of proteins at single-cell resolution, we can now visualize how tumors evolve in real-time within their tissue environment.”
One striking discovery was the identification of the NOVA2 protein, found only in invasive forms of the tumor and its metastases. “This protein could be part of a molecular ‘switch’ driving tumor cell invasion,” notes Schweizer.With these new targeted approaches, treatment can focus specifically on cancer cells while sparing healthy tissue. Clinical trials are still needed to confirm the safety and efficacy of the Milciclib-Mirvetuximab combination in humans, but for the approximately 5-10% of ovarian cancer patients diagnosed with LGSC, these advances offer renewed hope for more effective and less toxic treatment options.
Frequently Asked Questions
Low-Grade Serous Ovarian Cancer (LGSC) is a distinct subtype of ovarian cancer that accounts for approximately 5-10% of all epithelial ovarian cancers. Unlike the more common high-grade serous ovarian cancer, LGSC typically affects younger women, grows more slowly, but infiltrates deeply into healthy tissue. It’s characterized by resistance to standard platinum-based chemotherapy with response rates below 15%, and often leads to late recurrences. LGSC has fewer genetic mutations than high-grade ovarian cancer and follows a different developmental pathway.
The Milciclib-Mirvetuximab combination is a novel targeted therapy approach for treating LGSC. Milciclib is an oral drug that inhibits cyclin-dependent kinases, which blocks cancer cell division. Mirvetuximab is an antibody-drug conjugate that delivers a toxic payload specifically to cancer cells expressing the FOLR1 protein on their surface. This two-pronged approach targets specific molecular vulnerabilities in cancer cells rather than broadly attacking all rapidly dividing cells like traditional chemotherapy. In mouse models, this combination significantly reduced tumor burden in chemotherapy-resistant cancer.
Clinical trials showed that Mirvetuximab (a component of the combination therapy) achieved a 42.3% response rate compared to just 15.9% with standard chemotherapy—nearly triple the effectiveness. Overall survival increased from 12.75 months with chemotherapy to 16.46 months with Mirvetuximab. The duration of response averaged about 6.9 months, significantly better than the 3-4 months typically seen with standard treatments. These results represent a substantial improvement for a cancer type that has traditionally been difficult to treat.
Deep Visual Proteomics is a cutting-edge technology that combines modern microscopy, artificial intelligence, laser microdissection, and ultra-sensitive mass spectrometry to analyze proteins at an unprecedented level of detail. This technology allows researchers to create molecular maps showing exactly how tumors evolve within tissue environments. By mapping thousands of proteins at single-cell resolution, scientists can visualize cancer progression in real-time and identify previously hidden molecular patterns driving cancer development. This detailed understanding led to the identification of 16 potential drug targets for LGSC, including the NOVA2 protein which appears to be a key driver of tumor cell invasion.
Mirvetuximab received FDA approval in March 2024 for folate receptor-positive, platinum-resistant epithelial ovarian cancer. The treatment consists of an intravenous infusion once every three weeks until disease progression or unacceptable toxicity. However, cost remains a significant hurdle. Cost-effectiveness studies suggest a 70-73% reduction in price would be needed to meet standard cost-effectiveness thresholds in the U.S. healthcare system. While the Milciclib-Mirvetuximab combination showed promising results in research, clinical trials are still needed to confirm its safety and efficacy in humans before it becomes widely available as a combination therapy.
While the new targeted treatment approaches generally have fewer severe side effects than traditional chemotherapy, they still carry risks. With Mirvetuximab, the most common treatment-related adverse events include blurred vision, keratopathy (a disease of the cornea), and nausea. During clinical trials, these side effects led to dose delays in 33% of patients, dose reductions in 20%, and treatment discontinuations in 9%. Compared to standard chemotherapy, Mirvetuximab showed fewer adverse events of grade 3 or higher (41.7% vs. 54.1%) and fewer serious adverse events (23.9% vs. 32.9%). The FDA has included a boxed warning for ocular toxicity with Mirvetuximab, and patients require regular eye examinations during treatment.