Replace Animal Tests Act: Modernizing U.S. Regulatory Testing
The Current Reality
Hundreds of thousands of animals covered by federal reporting, and millions when including species not counted under USDA rules, are used annually in U.S. research and testing. Many experience pain, distress, and confinement in tests that exist primarily due to outdated regulatory requirements.
The problem: validated, non-animal testing methods are now available for certain applications. Scientifically advanced alternatives including organ-on-chip technologies, computational models, and human cell-based systems can be more predictive of human outcomes for specific endpoints and applications.
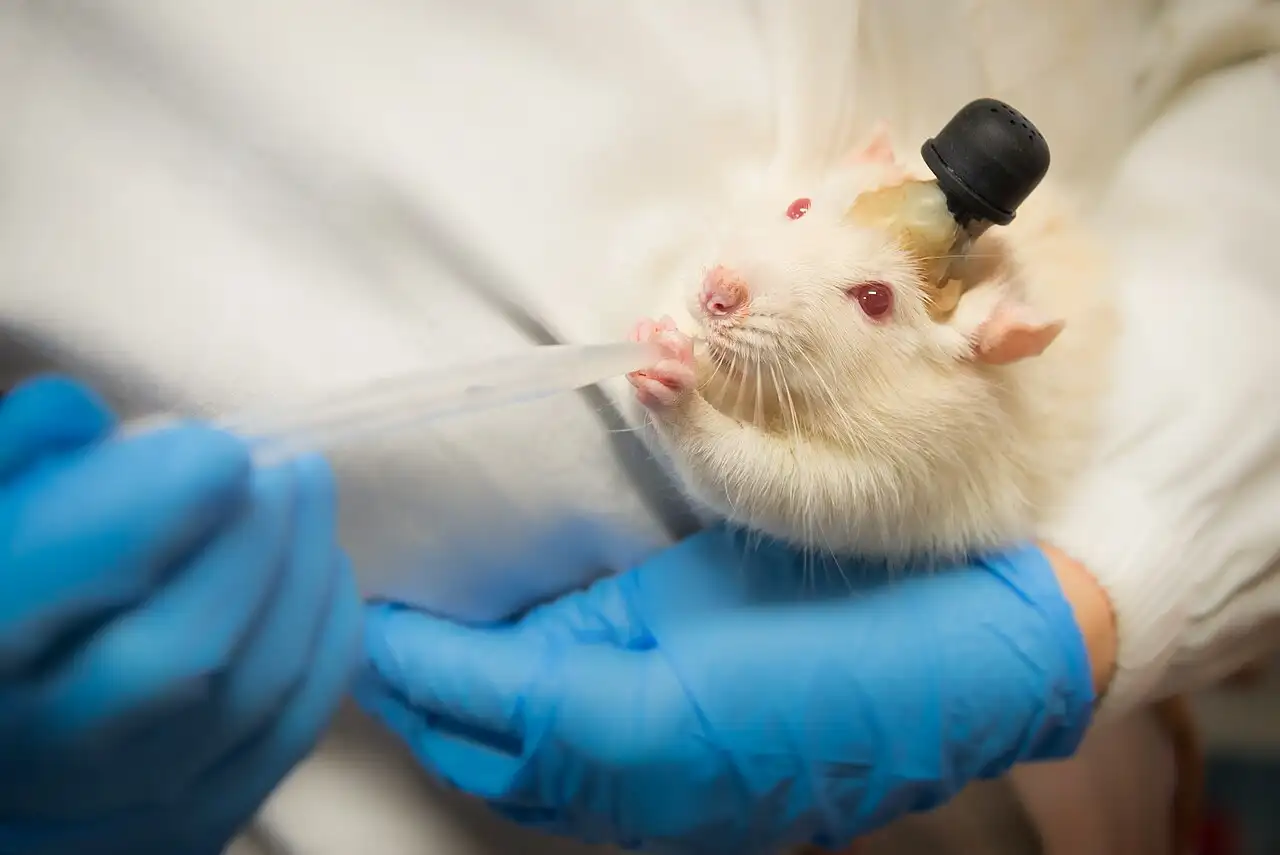
Laboratory rat in biomedical research. Photo: Wikimedia Commons (CC BY-SA 4.0)
A Gap Between Science and Regulation
In December 2025, Representatives Jared Moskowitz, Jan Schakowsky, and Shri Thanedar introduced the Replace Animal Tests Act of 2025 (H.R. 6660). This landmark legislation addresses a critical gap in federal policy: in many regulatory contexts, animal testing remains routine even where validated non-animal alternatives are available and accepted for specific uses.
The Replace Act would modernize U.S. regulatory testing standards by establishing a clear principle: when validated non-animal methods exist and are accepted by regulators, animal tests cannot be used instead. If enacted, the legislation would represent one of the most meaningful federal advances for animals in laboratories in years.
Animal protection and scientific advancement are not competing interests. They align when regulations match technological capability.
What the Replace Act Would Do
The bill establishes five core provisions to modernize regulatory testing:
Current Practice
Animal tests used in many regulatory contexts regardless of alternatives
No federal requirement to use validated non-animal methods where available
Limited transparency on animal use in regulatory testing
Replace Act Standard
Non-animal methods used when validated and accepted by agencies
Federal law requires agencies to prioritize alternatives
Annual public reporting on animal use, species, and purposes
The Five Core Provisions
The Replace Act would require major federal agencies—FDA, EPA, USDA, and Consumer Product Safety Commission—to implement these changes:
Ban on Unnecessary Animal Tests
Prohibit submission of animal test data to federal agencies when approved non-animal methods exist and are accepted by regulators.
Updated Guidance
Require federal agencies to issue guidance and update regulations reflecting the acceptability of non-animal testing methods.
Reduction Targets
Limit animal tests to circumstances where non-animal methods cannot meet testing requirements. Requires justification where non-animal alternatives are unavailable.
Public Transparency
Mandate annual public reporting on animal use, species involved, and testing purposes.
Minimization
When animal tests are necessary, minimize the number of animals used and document the rationale.
Federal Agency Coverage
The law applies to FDA, EPA, USDA, and Consumer Product Safety Commission.
Why This Matters Now
The scale of current animal testing and potential impact of modernization:
Testing Areas Identified for Modernization
Cruelty Free International identified ten regulatory testing procedures that continue across U.S. agencies despite validated non-animal alternatives existing for certain contexts:
Rabbit Pyrogen Test
Outdated bacterial detection method. Monocyte activation test provides faster, more accurate results for certain applications.
Skin Irritation Tests
Chemical application to animal skin when in vitro reconstruction methods are available for specific use cases.
Eye Irritation Tests
Draize test alternatives exist in validated in-vitro methods for certain endpoints.
Skin Sensitization Tests
Guinea pig maximization test replaced in many regulatory contexts by validated in-vitro and defined-approach methods.
Antibody Production
Animal use for antibody development when cell-based systems are available.
Batch Safety Tests
Multiple outdated animal-based safety verification procedures continue in use.
Understanding the Scientific Shift
Non-Animal Methods Now Available
Organ-on-a-Chip Technology: Engineered systems replicate human organ functions using actual human cells. They provide directly relevant testing data for certain applications and can be more predictive of human outcomes for specific endpoints.
3D Tissue Models: Advanced human cell-based three-dimensional systems replicate organ structure and function. Drugs are tested on actual human tissue without animal use for certain applications.
Computational Modeling & AI: AI and computational models can predict how chemicals behave in the human body using existing data and machine learning for certain testing purposes.
High-Throughput Screening: Automated systems test thousands of compounds simultaneously on human cell cultures, accelerating drug discovery without animal use for certain applications.
Human Biomonitoring and Real-World Data: Direct study of human populations provides complementary exposure data that can improve relevance alongside other methods.
Federal Agencies Already Moving Forward
The FDA released its April 2025 Roadmap to reduce animal testing in preclinical safety studies. The NIH announced in April 2025 it would prioritize grants incorporating organ-chips, organoids, or computational models. In July 2025, the NIH announced it will no longer develop new funding opportunities focused exclusively on animal models, with all new funding opportunities related to animal model systems now required to also support human-focused approaches such as clinical trials, real world data, or new approach methods.
“For too long, animal tests have continued not because they’re necessary, but because our rules haven’t kept pace with modern science. The Replace Animal Tests Act of 2025 finally closes that gap by giving agencies clear guidance and ensuring they stop relying on painful tests when better methods exist. No animal should have to suffer simply because our regulations haven’t caught up with the science.”
“Many people assume that once a non-animal alternative exists, animal testing stops—or at least becomes rare. Unfortunately, that’s not the case. Some animal tests continue, or even increase, despite the availability of modern options. The Replace Act supports both innovation and ethics by requiring the use of proven non-animal methods whenever they are available.”
Global Progress: International Leadership in Non-Animal Testing
The United States lags behind international standards in requiring non-animal methods. Other nations and regions are already mandating the use of alternatives where available:
European Union & UK
Pioneered requirements to use non-animal methods for decades. The Replace Act would align U.S. standards with EU and UK practices.
Brazil (2025)
Enacted a ban on the sale of cosmetics tested on animals. Hosted the 13th World Congress on Alternatives and Animal Use in the Life Sciences in August-September 2025.
Canada (2025)
Published a national strategy to promote replacement, reduction, and refinement in toxicity testing. Convened workshops bringing together government, academia, and industry stakeholders.
South Korea (2026)
Government-funded animal-free testing facility under construction, scheduled for completion in 2026. Will provide shared infrastructure for non-animal toxicity testing using human cells, 3D models, and AI-based tools.
U.S. States (Cosmetics)
Twelve states including California, New York, New Jersey, Illinois, and Virginia passed laws limiting animal testing in specific regulatory contexts or banning the sale of animal-tested cosmetics. These state actions demonstrate feasibility of implementation.
International Standards
OECD chemical safety testing standards have incorporated non-animal methods. EU environment policy prioritizes alternatives. UK government policy mandates reduction.
A Real-World Example: Montessa’s Journey
Montessa’s story illustrates both the scale of animal laboratory confinement and the possibility of change. In 1975, when she was just one year old, Montessa was brought to the Alamogordo Primate Facility, a federally operated laboratory in New Mexico. For 50 years, she lived in the facility. For her first 30 years there, she was used in harmful biomedical research experiments.
In 2015, after decades of advocacy and legal action, the federal government ended all experiments on federally owned chimpanzees and promised to move them to Chimp Haven, a sanctuary in Louisiana. Montessa and the other Alamogordo chimps, however, were kept at the laboratory for years. Following a federal court ruling in December 2022 that the NIH’s refusal to retire the chimps violated federal law, and continued advocacy pressure, Montessa finally arrived at Chimp Haven in 2025.
At age 51, Montessa is now experiencing sanctuary life for the first time. Her journey reflects both the problem—animals kept in laboratories due to outdated policies—and the solution: when legal and policy changes happen, decades of confinement can end and animals can experience freedom.
Recent Policy Developments
FDA Modernization Act 2.0 Signed Into Law
FDAMA 2.0 eliminated the statutory requirement for animal testing in all new drug applications. Allows pharmaceutical companies to use alternatives including cell-based assays and computational models.
FDA Releases Roadmap to Reduce Animal Testing
FDA published plan to reduce animal testing in preclinical safety studies, prioritizing organ-chips, human cell systems, and AI-driven toxicity modeling in Investigational New Drug submissions.
NIH Announces Changes to Funding Opportunities
NIH announced it will no longer develop new funding opportunities focused exclusively on animal models of human disease. All new funding opportunities related to animal model systems must now also support human-focused approaches such as clinical trials, real world data, or new approach methods.
Replace Animal Tests Act Introduced
H.R. 6660 introduced by Representatives Moskowitz, Schakowsky, and Thanedar. Aims to establish federal mandate for using non-animal methods when scientifically valid.
Current Moment: Congressional Consideration
Bill under consideration in Congress. Public advocacy and constituent pressure are critical for passage and implementation.
Why Congress Should Act
The Replace Animal Tests Act addresses three interconnected problems:
First, Animal Welfare: Hundreds of thousands of animals documented under federal rules, and millions when including other species, endure pain and confinement unnecessarily when better testing methods exist.
Second, Scientific Accuracy: For certain applications, non-animal methods can be more predictive of human outcomes than animal models. This means safer drugs for patients and fewer failed trials.
Third, Regulatory Competitiveness: U.S. regulations lag behind international standards. American companies developing innovative testing methods face regulatory uncertainty.
Good science and animal protection align when regulations catch up with technology. The Replace Act makes that alignment official federal policy.
Your Action Matters
Strong legislation requires constituent pressure. Lawmakers need to hear that this issue matters. One minute of action can help inform federal policy and support scientific modernization.
Moving Forward
The Replace Animal Tests Act represents alignment between federal policy and scientific capability. The bill brings standards into line with existing technological options, practices adopted by international regulators, and contemporary public values. The scientific foundation exists. Validated alternatives are available for many, but not all, regulatory testing purposes. Federal agencies have begun implementation through their own initiatives.
The Replace Act transforms these isolated efforts into consistent, mandated federal policy. Federal law would ensure that every agency uses validated non-animal methods when available—making progress systematic rather than agency-by-agency.
Public support will determine whether this legislation passes.