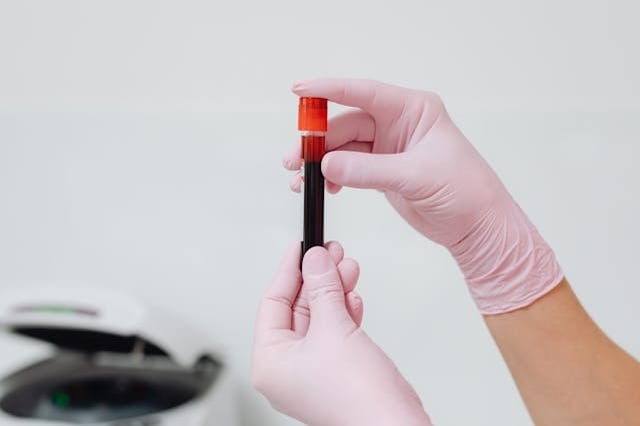
A revolutionary blood test that can detect 12 common cancers before symptoms appear is now being trialled on 8,000 NHS patients. The test, developed in Britain, uses artificial intelligence to identify cancer with high accuracy.
The miONCO-Dx test, created by UK biotech company Xgenera working with the University of Southampton, analyses microRNA in blood samples. These small molecules help regulate gene expression, and their abnormal levels can signal cancer’s presence.
Health Secretary Wes Streeting announced £2.4 million in government funding to advance the technology. He spoke about his personal experience with cancer and emphasized that early detection is crucial for survival.
Initial tests on 20,000 patients showed the test can detect cancer with over 99% accuracy. The test requires only a small blood sample to screen for a dozen dangerous cancers, including those affecting the lungs, stomach, prostate, esophagus, liver, bladder, ovaries, intestines, pancreas, and breast, along with certain sarcomas and brain tumors.
Improving Bowel Cancer Outcomes
The announcement coincided with the renaming of a research laboratory at the Francis Crick Institute in honour of Dame Deborah James, the bowel cancer campaigner who died from the disease aged 40 three years ago.
Statistics show bowel cancer ranks as the fourth most prevalent cancer in the UK, affecting more than 42,000 people annually and standing as the second leading cause of cancer mortality. Early detection drastically improves survival rates – 90% of patients survive when bowel cancer is caught at stage 1, compared to just 10% at stage 4.
Professor Sir Stephen Powis, NHS England’s National Medical Director, highlighted the test’s potential: “This blood test has the potential to help us detect bowel cancer earlier and reduce the need for invasive tests.”
Simpler, Less Invasive Testing
The test offers several advantages over current diagnostic methods. Patients currently undergo invasive procedures like colonoscopies and biopsies, which can be uncomfortable and resource-intensive.
Public Health Minister Ashley Dalton shared her experience with breast cancer, explaining that her diagnosis involved multiple complicated tests and appointments. She noted that a simple blood test could have made her diagnosis more efficient.
Similar Posts
The test is now entering the “validation and verification” stage. If successful, it could potentially be implemented more widely within the NHS.
Andy Shapanis, Chief Executive of Xgenera, emphasized that their goal is to create an affordable, rapid screening test suitable for widespread use. He pointed out that their technology is significantly less expensive than alternatives, making it highly scalable.
Professor Lucy Chappell, Chief Scientific Adviser at the Department of Health and Social Care, described innovations like the miONCO-Dx test as offering an “exciting new era in cancer detection” with the potential for quicker, easier, and more effective early detection.
Dame Deborah James’ Legacy
The Bowelbabe Laboratory, funded by Cancer Research UK, will focus on advancing understanding of bowel cancer and developing new tests and treatments.
Heather James, Dame Deborah James’ mother, reflected on her daughter’s legacy: “Deborah was quite a science geek really. Having known how much research she would go into for her own self and for other people, I think she would be so chuffed to see what her fundraising for the Babefund has gone towards.”
Michelle Mitchell, Chief Executive of Cancer Research UK, added: “She touched the lives of so many, and her legacy is supporting people affected by bowel cancer across the country.”

The blood test trial represents a significant step in the government’s broader strategy to reduce lives lost to major diseases. It forms part of the Plan for Change for the NHS, which the government says has already reduced waiting lists by over 200,000 since July.