Scientists at the University of Oxford have created a vaccine delivery system that could transform how we fight malaria worldwide. Their innovation tackles a major healthcare challenge – getting people to return for booster shots – by delivering both initial and booster doses in just one injection.
This breakthrough comes at a critical time. Malaria still kills about 600,000 people each year, with children under five in Africa bearing the heaviest burden. Current vaccines require multiple clinic visits, which is often impossible for families in remote areas with limited healthcare access.
“Reducing the number of clinic visits needed for full vaccination could make a major difference in communities where healthcare access is limited,” said Luca Bau, Senior Researcher from Oxford’s Institute of Biomedical Engineering.
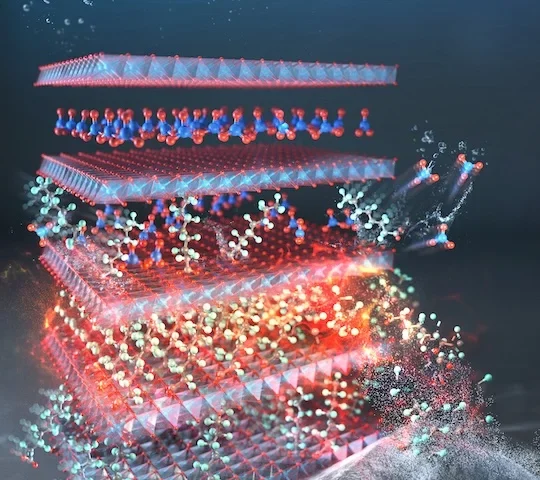
The technology works through tiny capsules made from a safe, biodegradable material called PLGA. These capsules, smaller than the width of a human hair, are injected alongside the first vaccine dose. While the initial vaccine begins working immediately, the capsules stay intact in the body until they’re programmed to release the booster dose weeks or months later.
“The microcapsules are precisely engineered to act as a tiny, timed-release vault,” explained Romain Guyon, who invented the technology. “This allows us to dictate exactly when the booster dose is released.”
In mouse testing, this single-shot approach using Oxford’s R21 malaria vaccine achieved 85% protection – comparable to the protection from traditional two-dose methods. Importantly, this protection lasted at least 11 weeks.
Similar Post
This represents a significant improvement over current options. Today, two WHO-approved malaria vaccines exist: Mosquirix (made by GSK) requires at least three doses and provides only 39% protection, while Oxford’s R21/Matrix-M needs two to three doses but offers better protection at about 77%.
The single-shot system addresses several practical challenges of vaccine delivery in low-resource settings. The capsules remain stable after refrigeration for 4-7 weeks, helping overcome the “cold chain” problem – keeping vaccines properly refrigerated in areas with unreliable electricity. Standard syringes can deliver the vaccine, and the manufacturing process is compatible with existing pharmaceutical methods, making large-scale production feasible.
“Our approach solves three of the biggest problems in delayed vaccine delivery: how to make it programmable, injectable, and scalable,” Guyon said.
The statistics highlight the need for better vaccination approaches. In 2022 alone, approximately 20.5 million children missed routine vaccinations worldwide, many due to difficulties accessing healthcare facilities multiple times.

The Oxford team is now preparing for human clinical trials, with findings published in Science Translational Medicine. Their work has attracted interest from pharmaceutical companies and global health organizations.
While initially focused on malaria, this technology could potentially be used for many other vaccines requiring multiple doses, including those for tuberculosis and HPV.
“This is the exciting first step in proving that it is possible to administer the full immunization complement through a single injection,” said Anita Milicic, Associate Professor at Oxford’s Jenner Institute. “We now turn to the next challenge: adapting and refining the approach for translation into the clinic.”
If successful in human trials, this innovation could become a crucial tool in global efforts to combat malaria and potentially many other diseases that currently require multi-dose vaccination schedules.