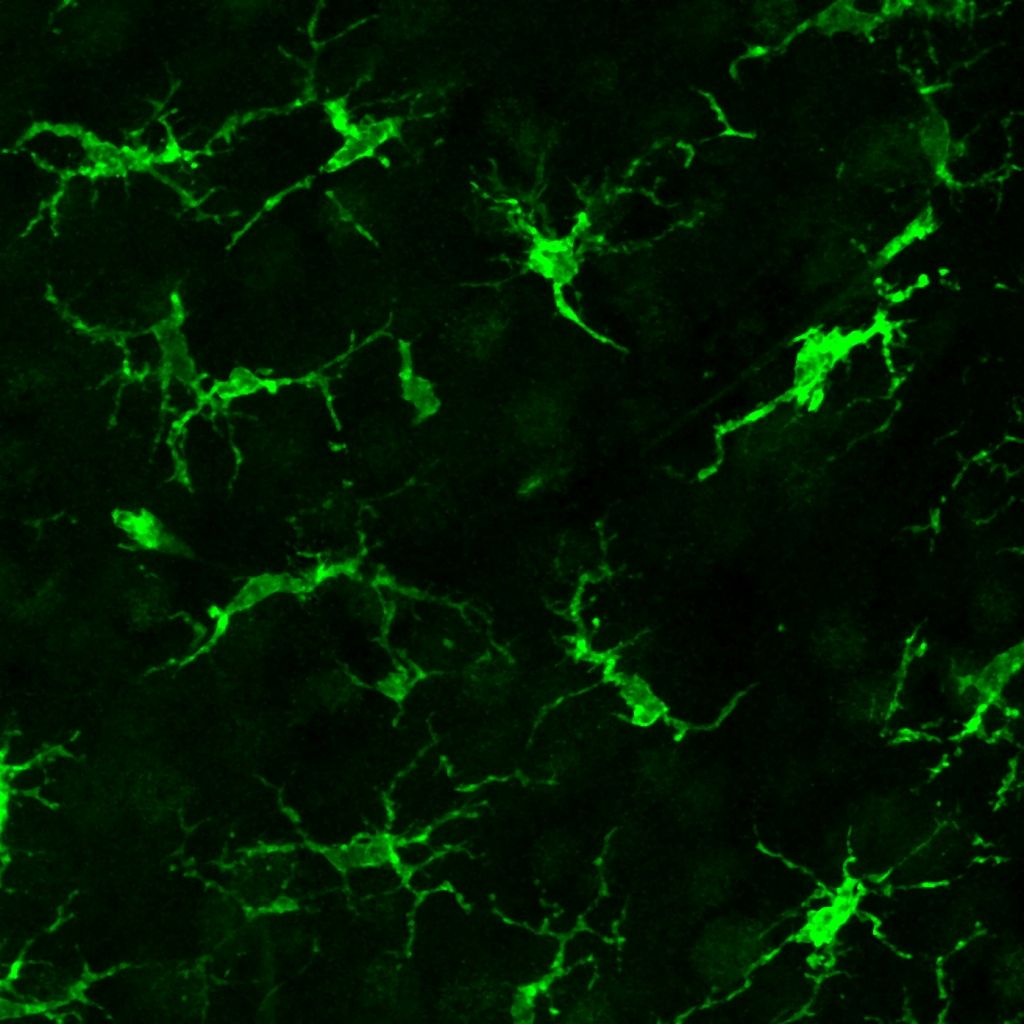
Stanford Medicine researchers have developed a groundbreaking method to replace defective brain immune cells with healthy ones, significantly slowing neurodegeneration in mice with a rare genetic disorder similar to Tay-Sachs disease.
The technique successfully replaced more than half of the brain’s microglia—specialized immune cells that clean up waste and protect the brain—with healthy donor cells that weren’t genetically matched to the recipient. This approach dramatically extended the animals’ lives and improved their symptoms.
“Using a specific sequence of steps, we were able to achieve nearly 100% incorporation of genetically healthy cells in the brains of the mice while avoiding both rejection and graft-versus-host disease,” said Dr. Marius Wernig, professor of pathology at Stanford and senior author of the study published in Nature on August 6, 2025.
The results were remarkable. While untreated mice with Sandhoff disease lived a maximum of 155 days, treated mice survived up to 250 days—when the experiment ended. The treated mice showed dramatically improved motor function and normal exploratory behaviors compared to untreated animals.
This advance tackles three major challenges that have hindered previous attempts at cell replacement therapy: achieving efficient brain-specific cell transplantation without toxic whole-body treatments, using non-genetically matched donor cells, and preventing immune rejection of the transplanted cells.
Similar Posts
The new method uses a carefully orchestrated sequence involving targeted brain irradiation, drugs to eliminate existing microglia, injection of donor microglia precursor cells directly into the brain, and medication to prevent immune rejection of the transplanted cells.
Researchers discovered an unexpected bonus—the transplanted microglia appeared to supply the missing enzyme to neighboring neurons, potentially explaining how the therapy rescued brain cells that weren’t directly replaced.
“This could be an important, unrecognized role for microglia: to supply lysosomal factors to the environment including neurons,” Wernig explained.
The approach offers particular promise because each component is already used clinically for other conditions, potentially accelerating the path to human trials. Additionally, because it doesn’t require genetically matched donors or genetic engineering of cells, it could someday become an “off-the-shelf” therapy that’s more accessible than custom treatments.
While initially targeting rare genetic disorders like Tay-Sachs and Sandhoff disease, which are fatal in early childhood, the researchers believe their approach could eventually help treat more common conditions.
“It’s possible that these lysosomal storage diseases are just an accelerated version of much more common neurodegenerative diseases like Alzheimer’s or Parkinson’s,” Wernig said. “If so, this therapy could be very relevant not just for a small subset of children, but for many, many more people.”