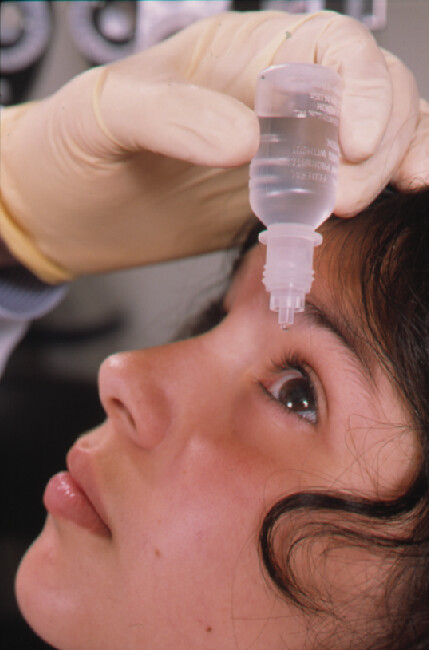
The FDA discovered manufacturing problems at a facility making popular eye drops, leading to a massive recall affecting millions of packages nationwide. AvKare, the distributor, pulled five different eye care products from store shelves after an audit found the manufacturing company couldn’t guarantee the products were sterile.
BRS Analytical Service, the manufacturer, failed to meet federal manufacturing standards during the FDA inspection. The agency found what they called “a lack of assurance of sterility” – a serious concern for products applied directly to the eye. AvKare acknowledged these problems “may lead to products of unacceptable quality” and warned that “it is not possible to rule out patient risks.”
What Products Were Recalled
Five eye care products shipped between May 2023 and April 2025 are affected. The recall includes:
- Artificial Tears Ophthalmic Solution (13,872 cases)
- Carboxymethylcellulose Sodium Ophthalmic Gel 1% (1,610 cases)
- Carboxymethylcellulose Sodium Ophthalmic Solution1 0.5% (32,876 cases)
- Lubricant Eye Drops Solution (13,104 cases)
- Polyvinyl Alcohol Ophthalmic Solution 1.4% (14,333 cases)
Each product has a specific NDC number on the package that helps identify if you have the recalled version. The total recall covers approximately 75,000 cases containing 1.8 million individual packages.
Why This Matters
Eye drops and artificial tears go directly onto the sensitive tissues of your eye. If they’re not properly sterilized during manufacturing, harmful bacteria could grow inside the bottle. Using contaminated eye drops can cause serious infections that might damage your vision or even lead to blindness in severe cases.
The FDA classified this as a Class II recall, meaning the products could cause temporary or reversible health problems. While the chance of serious consequences is considered remote, the agency can’t rule out the possibility entirely.
What You Should Do
Stop using any of these AvKare eye products immediately. Check the NDC number on your package against the list above. Even if you haven’t experienced problems, don’t take the risk.
If you have one of these products, AvKare wants you to return it for a full refund. Fill out their “Quality to Return” form and either fax it to 931-292-6229 or email it to customerservice@avkare.com. They’ll send you a return authorization and cover all shipping costs.
Similar Posts
The Bigger Picture
This recall happened during allergy season when many people rely on eye drops for relief from itchy, watery eyes. The timing makes the safety issue even more concerning since demand for these products typically increases during spring months.
The FDA routinely audits drug manufacturing facilities to ensure they follow strict guidelines called Current Good Manufacturing Practices (cGMP). These rules help guarantee that medicines are consistently made to the same quality standards. When companies don’t follow these practices, it raises red flags about product safety.
Manufacturing deviations can include problems with equipment, processes, or environmental controls. In this case, the specific issues that led to the sterility concerns weren’t detailed, but they were serious enough to trigger a nationwide recall.
No Illnesses Reported Yet
As of now, no one has reported getting sick from these eye drops. However, the FDA and AvKare issued the recall as a precaution. If you’ve been using any of these products and notice eye redness, pain, swelling, discharge, or vision changes, contact your doctor right away.
Getting Updates
For the latest information about the recall, check the official AvKare website or the FDA’s recall database. The company continues working with federal regulators to remove all affected products from stores and resolve the manufacturing issues that caused the problem.
This recall serves as a reminder that even over-the-counter products face strict safety standards. When those standards aren’t met, regulatory agencies act quickly to protect public health – which is exactly what happened here.