Scientists have uncovered a surprising insight into why brain cells die in Parkinson’s disease – they might simply be working too hard. The finding could open new doors for treating this debilitating condition that affects over 8 million people worldwide.
Researchers at the Gladstone Institute for Neurological Disease focused on dopamine neurons – brain cells essential for controlling body movement that progressively die in Parkinson’s disease. Their study, published in the journal eLife, suggests these neurons might burn out from excessive activity.
“An overarching question in the Parkinson’s research field has been why the cells that are most vulnerable to the disease die,” said Dr. Ken Nakamura, who led the study. “Answering that question could help us understand why the disease occurs and point toward new ways to treat it.”

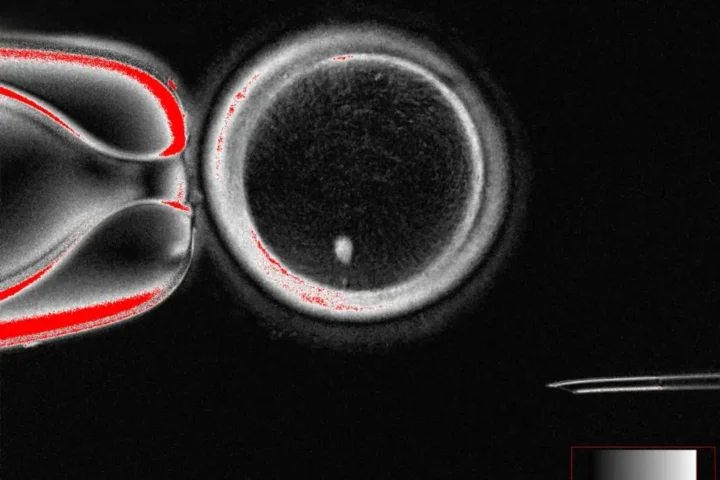
The research team tested this theory by creating genetically modified mice whose dopamine neurons could be artificially stimulated. By adding a drug called clozapine-N-oxide to the animals’ drinking water, they triggered continuous activation of these brain cells.
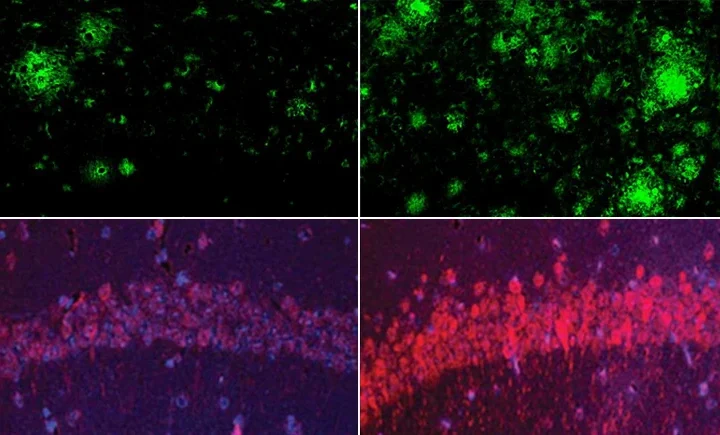
The results were striking. Within days, the mice showed disrupted activity patterns. After just one week, the long extensions (axons) of dopamine neurons began to degenerate. By the one-month mark, the neurons themselves started dying.
Similar Posts
Importantly, the damage specifically affected neurons in the substantia nigra – the brain region responsible for movement control that deteriorates in Parkinson’s patients. Meanwhile, dopamine neurons in areas controlling motivation and emotions remained largely unharmed, mirroring what happens in human Parkinson’s disease.
When the scientists examined what was happening inside these overworked neurons, they found changes in calcium levels and altered expression of genes related to dopamine production. Similar patterns appeared in brain samples from people with early-stage Parkinson’s disease.

“In response to chronic activation, we think the neurons may try to avoid excessive dopamine – which can be toxic – by decreasing the amount of dopamine they produce,” explained Katerina Rademacher, first author of the study. “Over time, the neurons die, eventually leading to insufficient dopamine levels in the brain areas that support movement.”
The researchers suggest a vicious cycle might be at work: as some overactive neurons die, the remaining ones work even harder to compensate, accelerating their own decline – similar to lightbulbs burning too brightly before burning out.
While scientists have previously proposed various reasons for dopamine neuron death in Parkinson’s – from malfunctioning cellular powerhouses (mitochondria) to harmful protein clumps – this new finding adds another piece to the puzzle.
The discovery raises exciting possibilities for treatment. “It raises the exciting possibility that adjusting the activity patterns of vulnerable neurons with drugs or deep brain stimulation could help protect them and slow disease progression,” said Nakamura.
This research gains urgency considering Parkinson’s growing impact. According to global health statistics, the prevalence of Parkinson’s has doubled over the past 25 years, with approximately 8.5 million people now affected worldwide.
While the study doesn’t reveal why these neurons become hyperactive in the first place, the researchers suggest multiple factors could be involved, including genetic predisposition and environmental triggers. Further research is needed to determine if this neuronal burnout is a primary cause of Parkinson’s or a consequence of the disease process.
The findings suggest that treatments focused on modulating brain activity – not just replacing lost dopamine – might slow Parkinson’s progression rather than merely treating symptoms.