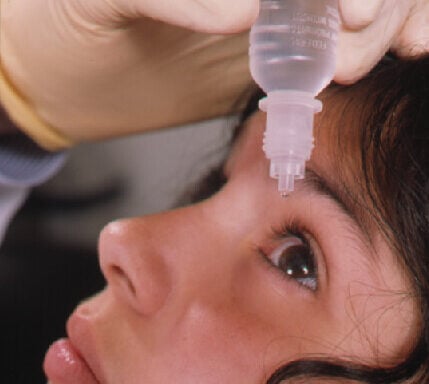
The FDA discovered manufacturing problems at a facility making popular eye drops, leading to a massive recall affecting millions of packages nationwide. AvKare, the distributor, pulled five different eye care products from store shelves after an audit found the manufacturing company couldn’t guarantee the products were sterile. BRS Analytical Service, the manufacturer, failed to meet federal manufacturing standards during the FDA inspection. The agency found what they called “a lack of assurance of sterility” – a serious concern for products applied directly to the eye. AvKare acknowledged these problems “may lead to products of unacceptable quality” and warned that “it … Continue reading AvKare Eye Drops Recalled: 1.8 Million Packages Pulled After FDA Finds Sterility Failures in Manufacturing Plant